Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakamura, Shoji; Shibahara, Yuji*; Kimura, Atsushi; Endo, Shunsuke; Shizuma, Toshiyuki*
Journal of Nuclear Science and Technology, 60(9), p.1133 - 1142, 2023/09
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)In recent years, research has been advanced on lead-cooled fast reactors and accelerator drive systems, and it is required to improve the accuracy of the neutron capture cross section of Pb isotopes. Although 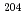 Pb has a small natural abundance, it is of importance because it produces the long-lived radionuclide
Pb has a small natural abundance, it is of importance because it produces the long-lived radionuclide 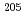 Pb (17.3 million years) by neutron capture reaction. However, it is difficult to measure its cross section by a conventional activation method using a nuclear reactor because the induced radioactivity of
Pb (17.3 million years) by neutron capture reaction. However, it is difficult to measure its cross section by a conventional activation method using a nuclear reactor because the induced radioactivity of 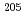 Pb is weak. Hence, the cross-section measurement was performed by applying mass spectrometry. This presentation gives the details of the experiment and the results obtained in the neutron capture cross-section measurement of
Pb is weak. Hence, the cross-section measurement was performed by applying mass spectrometry. This presentation gives the details of the experiment and the results obtained in the neutron capture cross-section measurement of 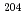 Pb using mass spectroscopy.
Pb using mass spectroscopy.
 Sr analysis of environmental samples by ion-laser interaction mass spectrometry
Sr analysis of environmental samples by ion-laser interaction mass spectrometryHonda, Maki; Martschini, M.*; Marchhart, O.*; Priller, A.*; Steier, P.*; Golser, R.*; Sato, Tetsuya; Tsukada, Kazuaki; Sakaguchi, Aya*
Analytical Methods, 14(28), p.2732 - 2738, 2022/07
Times Cited Count:2 Percentile:45.92(Chemistry, Analytical)The sensitive  Sr analysis with accelerator mass spectrometry (AMS) was developed for the advances of environmental radiology. One advantage of AMS is the ability to analyze various environmental samples with
Sr analysis with accelerator mass spectrometry (AMS) was developed for the advances of environmental radiology. One advantage of AMS is the ability to analyze various environmental samples with  Sr/
Sr/ Sr atomic ratios of 10
Sr atomic ratios of 10 in a simple chemical separation. Three different IAEA samples with known
in a simple chemical separation. Three different IAEA samples with known  Sr concentrations (moss-soil, animal bone, Syrian soil: 1 g each) were analyzed to assess the validity of the chemical separation and the AMS measurement. The
Sr concentrations (moss-soil, animal bone, Syrian soil: 1 g each) were analyzed to assess the validity of the chemical separation and the AMS measurement. The  Sr measurements were conducted on the AMS system combined with the Ion Laser InterAction MasSpectrometry (ILIAMS) setup at the University of Vienna, which has excellent isobaric separation performance. The isobaric interference of
Sr measurements were conducted on the AMS system combined with the Ion Laser InterAction MasSpectrometry (ILIAMS) setup at the University of Vienna, which has excellent isobaric separation performance. The isobaric interference of  Zr in the
Zr in the  Sr AMS was first removed by chemical separation. The separation factor of Zr in two-step column chromatography with Sr resin and anion exchange resin was 10
Sr AMS was first removed by chemical separation. The separation factor of Zr in two-step column chromatography with Sr resin and anion exchange resin was 10 . The
. The  Zr remaining in the sample was removed by ILIAMS effectively. This simple chemical separation achieved a limit of detection
Zr remaining in the sample was removed by ILIAMS effectively. This simple chemical separation achieved a limit of detection  0.1 mBq in the
0.1 mBq in the  Sr AMS, which is lower than typical
Sr AMS, which is lower than typical  -ray detection. The agreement between AMS measurements and nominal values for the
-ray detection. The agreement between AMS measurements and nominal values for the  Sr concentrations of IAEA samples indicated that the new highly-sensitive
Sr concentrations of IAEA samples indicated that the new highly-sensitive  Sr analysis in the environmental samples with AMS is reliable even for high matrix samples of soil and bone.
Sr analysis in the environmental samples with AMS is reliable even for high matrix samples of soil and bone.
Hain, K.*; Martschini, M.*; G lce, F.*; Honda, Maki; Lachner, J.*; Kern, M.*; Pitters, J.*; Quinto, F.*; Sakaguchi, Aya*; Steier, P.*; et al.
lce, F.*; Honda, Maki; Lachner, J.*; Kern, M.*; Pitters, J.*; Quinto, F.*; Sakaguchi, Aya*; Steier, P.*; et al.
Frontiers in Marine Science (Internet), 9, p.837515_1 - 837515_17, 2022/03
Times Cited Count:12 Percentile:96.12(Environmental Sciences)Recent major advances in accelerator mass spectrometry (AMS) at the Vienna Environmental Research Accelerator (VERA) regarding detection efficiency and isobar suppression have opened possibilities for the analysis of additional long-lived radionuclides at ultra-low environmental concentrations. These radionuclides, including  U,
U,  Cs,
Cs,  Tc and
Tc and  Sr, will become important for oceanographic tracer application due to their generally conservative behavior in ocean water. In particular, the isotope ratios
Sr, will become important for oceanographic tracer application due to their generally conservative behavior in ocean water. In particular, the isotope ratios  U/
U/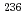 U and
U and  Cs/
Cs/ Cs have proven to be powerful fingerprints for emission source identification as they are not affected by elemental fractionation. Improved detection efficiencies allowed us to analyze all major long-lived actinides, i.e.
Cs have proven to be powerful fingerprints for emission source identification as they are not affected by elemental fractionation. Improved detection efficiencies allowed us to analyze all major long-lived actinides, i.e. 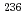 U,
U,  Np,
Np, 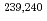 Pu,
Pu,  Am as well as the very rare
Am as well as the very rare  U, in the same 10 L water samples of an exemplary depth profile from the northwest Pacific Ocean. Especially for
U, in the same 10 L water samples of an exemplary depth profile from the northwest Pacific Ocean. Especially for  Sr analysis, our new approach has already been validated for selected reference materials (e.g. IAEA-A-12) and is ready for application in oceanographic studies. We estimate that a sample volume of only (1-3) L ocean water is sufficient for
Sr analysis, our new approach has already been validated for selected reference materials (e.g. IAEA-A-12) and is ready for application in oceanographic studies. We estimate that a sample volume of only (1-3) L ocean water is sufficient for  Sr as well as
Sr as well as  Cs analysis, respectively.
Cs analysis, respectively.
Miyajima, Yusuke*; Saito, Ayaka*; Kagi, Hiroyuki*; Yokoyama, Tatsunori; Takahashi, Yoshio*; Hirata, Takafumi*
Geostandards and Geoanalytical Research, 45(1), p.189 - 205, 2021/03
Times Cited Count:4 Percentile:21.77(Geochemistry & Geophysics)Uncertainty for elemental and isotopic analyses of calcite by LA-ICP-MS is largely controlled by the homogeneity of the reference materials (RMs) used for normalization and validation. In order to produce calcite RMs with homogeneous elemental and isotopic compositions, we incorporated elements including U, Pb, and rare earth elements into calcite through heat- and pressure-induced crystallization from amorphous calcium carbonate that was precipitated from element-doped reagent solution. X-ray absorption spectra showed that U was present as U(VI) in the synthesized calcite, probably with a different local structure from that of aqueous uranyl ions. The uptake rate of U by our calcite was higher in comparison to synthetic calcite of previous studies. Variations of element mass fractions in the calcite were better than 12% 2RSD, mostly within 7%. The 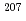 Pb/
Pb/ Pb ratio in the calcite showed
Pb ratio in the calcite showed  1% variations, while the
1% variations, while the  U/
U/ Pb ratio showed 3-24% variations depending on element mass fractions. Using the synthetic calcite as primary RMs, we could date a natural calcite RM, WC-1, with analytical uncertainty as low as
Pb ratio showed 3-24% variations depending on element mass fractions. Using the synthetic calcite as primary RMs, we could date a natural calcite RM, WC-1, with analytical uncertainty as low as  3%. The method presented can be useful to produce calcite with controlled and homogeneous element mass fractions, and is a promising alternative to natural calcite RMs for U-Pb geochronology.
3%. The method presented can be useful to produce calcite with controlled and homogeneous element mass fractions, and is a promising alternative to natural calcite RMs for U-Pb geochronology.
 Cs
CsShibahara, Yuji*; Nakamura, Shoji; Uehara, Akihiro*; Fujii, Toshiyuki*; Fukutani, Satoshi*; Kimura, Atsushi; Iwamoto, Osamu
Journal of Radioanalytical and Nuclear Chemistry, 325(1), p.155 - 165, 2020/07
Times Cited Count:8 Percentile:71.58(Chemistry, Analytical)The measurements of isotopic ratios of Cs samples by thermal ionization mass spectrometry were performed for the analysis of their samples used to evaluate nuclear data obtained for  Cs. To obtain a high intensity and stable ion beam, the effects of additive agents on the ionization of Cs were examined. The effect of silicotungstic acid on the ionization of Cs was the largest among the additive agents studied in the present study, while the silicotungstic acid also showed the largest isobaric interference of polyatomic ions. It was demonstrated that as small as 2
Cs. To obtain a high intensity and stable ion beam, the effects of additive agents on the ionization of Cs were examined. The effect of silicotungstic acid on the ionization of Cs was the largest among the additive agents studied in the present study, while the silicotungstic acid also showed the largest isobaric interference of polyatomic ions. It was demonstrated that as small as 2 10
10 g of a Cs sample was sufficient to achieve the analytical precision required to measure the
g of a Cs sample was sufficient to achieve the analytical precision required to measure the  Cs/
Cs/ Cs ratio in the case where an additive agent of TaO/glucose was employed. After examining of the analytical conditions, such as the interference effect due to Ba, the measurements of the isotopic ratios of two Cs samples used in our study using TIMS were conducted, and it was discussed how much the ratios contributed to evaluation of the neutron capture cross-section of
Cs ratio in the case where an additive agent of TaO/glucose was employed. After examining of the analytical conditions, such as the interference effect due to Ba, the measurements of the isotopic ratios of two Cs samples used in our study using TIMS were conducted, and it was discussed how much the ratios contributed to evaluation of the neutron capture cross-section of  Cs.
Cs.
Miwa, Kazuji; Obata, Hajime*; Suzuki, Takashi
Journal of Nuclear Science and Technology, 57(5), p.537 - 545, 2020/05
Times Cited Count:2 Percentile:21.58(Nuclear Science & Technology)This study investigated the vertical distribution of Iodine-129 ( I) which is mainly produced by European nuclear reprocessing plants in the Chukchi Sea and Bering Sea.
I) which is mainly produced by European nuclear reprocessing plants in the Chukchi Sea and Bering Sea.  I was found to be distributed almost uniformly in fallout level, and an increasing in
I was found to be distributed almost uniformly in fallout level, and an increasing in  I concentration levels caused by high
I concentration levels caused by high  I water inflow from the Atlantic Ocean was not observed. Additionally, we revealed the vertical distribution of iodide, one chemical form of iodine, from the Bering Shelf area to the Chukchi Sea for the first time. The increasing tendency of iodide near sea bottom was observed.
I water inflow from the Atlantic Ocean was not observed. Additionally, we revealed the vertical distribution of iodide, one chemical form of iodine, from the Bering Shelf area to the Chukchi Sea for the first time. The increasing tendency of iodide near sea bottom was observed.
Nakamura, Shoji; Shibahara, Yuji*; Kimura, Atsushi; Iwamoto, Osamu; Uehara, Akihiro*; Fujii, Toshiyuki*
Journal of Nuclear Science and Technology, 57(4), p.388 - 400, 2020/04
Times Cited Count:3 Percentile:31.89(Nuclear Science & Technology)The thermal-neutron capture cross-section (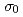 ) and resonance integral(I
) and resonance integral(I ) were measured for the
) were measured for the  Cs(n,
Cs(n, )
)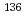 Cs reaction by an activation method and mass spectrometry. We used
Cs reaction by an activation method and mass spectrometry. We used  Cs contained as an impurity in a normally available
Cs contained as an impurity in a normally available  Cs standard solution. An isotope ratio of
Cs standard solution. An isotope ratio of  Cs and
Cs and  Cs in a standard
Cs in a standard  Cs solution was measured by mass spectrometry to quantify
Cs solution was measured by mass spectrometry to quantify  Cs. The analyzed
Cs. The analyzed  Cs samples were irradiated at the hydraulic conveyer of the research reactor in Institute for Integral Radiation and Nuclear Science, Kyoto University. Wires of Co/Al and Au/Al alloys were used as neutron monitors to measure thermal-neutron fluxes and epi-thermal Westcott's indices at an irradiation position. A gadolinium filter was used to measure the
Cs samples were irradiated at the hydraulic conveyer of the research reactor in Institute for Integral Radiation and Nuclear Science, Kyoto University. Wires of Co/Al and Au/Al alloys were used as neutron monitors to measure thermal-neutron fluxes and epi-thermal Westcott's indices at an irradiation position. A gadolinium filter was used to measure the 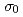 , and a value of 0.133 eV was taken as the cut-off energy. Gamma-ray spectroscopy was used to measure induced activities of
, and a value of 0.133 eV was taken as the cut-off energy. Gamma-ray spectroscopy was used to measure induced activities of  Cs,
Cs, 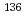 Cs and monitor wires. On the basis of Westcott's convention, the
Cs and monitor wires. On the basis of Westcott's convention, the 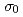 and I
and I values were derived as 8.57
values were derived as 8.57 0.25 barn, and 45.3
0.25 barn, and 45.3 3.2 barn, respectively. The
3.2 barn, respectively. The 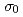 obtained in the present study agreed within the limits of uncertainties with the past reported value of 8.3
obtained in the present study agreed within the limits of uncertainties with the past reported value of 8.3 0.3 barn.
0.3 barn.
Nakamura, Shoji; Kimura, Atsushi; Iwamoto, Osamu; Shibahara, Yuji*; Uehara, Akihiro*; Fujii, Toshiyuki*
KURNS Progress Report 2018, P. 106, 2019/08
Under the ImPACT project, the neutron capture cross-section measurements of Cesium-135 ( Cs) among the long-lived fission products have been performed at Kyoto University. This paper reports measurements of the thermal-neutron capture cross-section of
Cs) among the long-lived fission products have been performed at Kyoto University. This paper reports measurements of the thermal-neutron capture cross-section of  Cs at the Kyoto University Research Reactor (KUR).
Cs at the Kyoto University Research Reactor (KUR).
 500
500 C) under electric field
C) under electric fieldBaba, Yuji; Shimoyama, Iwao
e-Journal of Surface Science and Nanotechnology (Internet), 16, p.53 - 59, 2018/03
Surface ionization for cesium chloride and Cs-adsorbed soil has been investigated. For cesium chloride, neutral cesium was desorbed around 645 C which is close to the melting point of cesium. While Cs
C which is close to the melting point of cesium. While Cs ion was desorbed from 400
ion was desorbed from 400 C. The ratio of desorbed ions and neutrals (Cs
C. The ratio of desorbed ions and neutrals (Cs /Cs
/Cs ) has a maximum around 410
) has a maximum around 410  C. Temperature dependence of Cs
C. Temperature dependence of Cs /Cs
/Cs was analyzed using Saha-Langmuir equation, As a result, it was found that the temperature maximum is due to the changes of the surface work function induced by the phase transition of CsCl.
was analyzed using Saha-Langmuir equation, As a result, it was found that the temperature maximum is due to the changes of the surface work function induced by the phase transition of CsCl.
Esaka, Fumitaka
Analytical Sciences, 33(10), p.1097 - 1098, 2017/10
Times Cited Count:4 Percentile:83.2(Chemistry, Analytical)Inductively coupled plasma-mass spectrometry (ICP-MS) is widely used in various fields such as environmental, geological, and clinical sciences. In this report, recent advances of the ICP-MS analysis and expected applications are described.
Shibahara, Yuji*; Hori, Junichi*; Takamiya, Koichi*; Fujii, Toshiyuki*; Fukutani, Satoshi*; Sano, Tadafumi*; Harada, Hideo
EPJ Web of Conferences, 146, p.03028_1 - 03028_4, 2017/09
Times Cited Count:2 Percentile:78.04(Nuclear Science & Technology)Esaka, Fumitaka; Suzuki, Daisuke; Yomogida, Takumi; Magara, Masaaki
Analytical Methods, 8(7), p.1543 - 1548, 2016/02
Times Cited Count:8 Percentile:50.61(Chemistry, Analytical)The isotope ratio analysis of individual uranium particles in environmental samples taken at nuclear facilities is important to clarify their origins for nuclear safeguards. In the present study, automated particle screening was used to select uranium particles prior to precise isotope ratio analysis by thermal ionization mass spectrometry (TIMS). As a result, molecular ion interferences on the uranium mass region were able to be almost completely avoided in the analysis of real inspection samples using APM-TIMS. Therefore, the performance of APM-TIMS was sufficient for obtaining isotope ratio data of individual particles without molecular ion interferences.

 ions
ionsNakajima, Kaoru*; Nagano, Kengo*; Suzuki, Motofumi*; Narumi, Kazumasa; Saito, Yuichi; Hirata, Koichi*; Kimura, Kenji*
Applied Physics Letters, 104(11), p.114103_1 - 114103_4, 2014/03
Times Cited Count:6 Percentile:26.85(Physics, Applied)
Nakajima, Kunihisa; Arai, Yasuo; Yamashita, Toshiyuki
Journal of Physics and Chemistry of Solids, 66(2-4), p.639 - 642, 2005/02
Times Cited Count:2 Percentile:12.62(Chemistry, Multidisciplinary)PuCd intermetallic compound was prepared by heating pure Pu and Cd metals at about 950K. PuCd
intermetallic compound was prepared by heating pure Pu and Cd metals at about 950K. PuCd was found to be a prototype of CdI
was found to be a prototype of CdI by means of powder X-ray diffractometry. Mass-spectrometric experiment was performed in the temperature range of 650-770K. It was found that the vapor pressures of Cd over PuCd
by means of powder X-ray diffractometry. Mass-spectrometric experiment was performed in the temperature range of 650-770K. It was found that the vapor pressures of Cd over PuCd +Pu were three to five orders of magnitude lower than those over Cd in this temperature range. From these vapor pressures, Gibbs free energy of formation of PuCd
+Pu were three to five orders of magnitude lower than those over Cd in this temperature range. From these vapor pressures, Gibbs free energy of formation of PuCd was evaluated.
was evaluated.
Miyabe, Masabumi; Kato, Masaaki; Oba, Masaki; Wakaida, Ikuo; Watanabe, Kazuo
JAERI-Tech 2004-065, 19 Pages, 2004/10
In nuclear waste materials there are various radionuclides to which standard analytical techniques are difficult to be applied. We are developing an analytical technique where such nuclides are ionized and mass-analyzed using diode laser based multi-step RIMS technique. The diode laser, however, has one drawback, i.e. its oscillation wavelength is readily drifted by acoustic, electric and optical noise, and thus the laser without frequency stabilization is not suitable for the analysis. In this study, we have developed (1) the diode laser whose frequency is stabilized to an intense absorption line of Rb by Zeeman effect and (2) the stabilization system where diode lasers for 3-step ionization of Ca are locked to the Rb-stabilized laser using a Fabry-perot interferometer. Additionally, to evaluate overall frequency stability of the stabilization system, fluctuations in the photoion and fluorescence signals arising from 3-step RIMS of Ca were simultaneously observed.
Miyabe, Masabumi; Oda, Koichi*; Oba, Masaki; Kato, Masaaki; Wakaida, Ikuo; Watanabe, Kazuo
JAERI-Tech 2004-064, 33 Pages, 2004/10
In nuclear waste materials there are various radionuclides to which standard analytical techniques are difficult to be applied. We are developing an analytical technique where such nuclides are analyzed using multi-step resonance ionization mass spectrometry. In this study, we have developed an external cavity diode laser applicable to the analysis. The wavelength and output power dependence on injection current and temperature were investigated for various types of laser diodes. Based on the data, we have obtained a suitable condition to operate the ECDL in stable single-mode oscillation, so that a continuous scanning range of about 100 GHz was realized. Additionaly, to evaluate the bandwidth of the developed ECDL, we have performed Doppler-free spectroscopy. The reasonable agreement of the measured isotope shift and HFS splitting with the reported values demonstrated that the developed ECDL is applicable to a precise laser spectroscopy as well as a laser trace analysis.
Baba, Yuji
Hyomen Kagaku No Kiso To Oyo, p.751 - 755, 2004/06
no abstracts in English
 /Si(001) system observed by SiO mass spectrometry and synchrotron radiation photoemission spectroscopy
/Si(001) system observed by SiO mass spectrometry and synchrotron radiation photoemission spectroscopyTeraoka, Yuden; Moritani, Kosuke; Yoshigoe, Akitaka
Applied Surface Science, 216(1-4), p.8 - 14, 2003/06
Times Cited Count:6 Percentile:35.62(Chemistry, Physical)The experiments concerning the oxidation of Si(001) were performed at the surface reaction analysis apparatus, installed at the beamline BL23SU in the SPring-8. The SiO desorb remarkably at surface temperature of 1000 K. The desorption yield increased with increasing the incident energy of O . On the other hand, the desorption yield increased with decreasing the incident energy in the temperature region lower than 1000 K. Oxygen uptake curves observed by O-1s photoemission measurements corresponded to the SiO desorption features. These facts reveal that the passive oxidation coexists with the SiO desorption in the temperature region from 900 K to 1000 K.
. On the other hand, the desorption yield increased with decreasing the incident energy in the temperature region lower than 1000 K. Oxygen uptake curves observed by O-1s photoemission measurements corresponded to the SiO desorption features. These facts reveal that the passive oxidation coexists with the SiO desorption in the temperature region from 900 K to 1000 K.
Nakajima, Kunihisa; Arai, Yasuo
Journal of Nuclear Materials, 317(2-3), p.243 - 251, 2003/05
Times Cited Count:3 Percentile:25.79(Materials Science, Multidisciplinary)The Knudsen effusion mass-spectrometric measurement of pure UO (s) are carried out at 1673, 1773 and 1873K to evaluate
(s) are carried out at 1673, 1773 and 1873K to evaluate  G
G
 (UO
(UO ,g) as well as to measure the partial pressures of UO
,g) as well as to measure the partial pressures of UO (g) and O
(g) and O (g) over UO
(g) over UO (s) as function of the O/U ratio. It was found that the partial pressures of O
(s) as function of the O/U ratio. It was found that the partial pressures of O (g) over UO
(g) over UO (s) almost agree with the experimental data reported in the past and the values derived from the empirical equation given by Nakamura and Fujino. Further, it was found that the values of
(s) almost agree with the experimental data reported in the past and the values derived from the empirical equation given by Nakamura and Fujino. Further, it was found that the values of  G
G
 (UO
(UO ,g) obtained in this study are in good agreement with the recommended values.
,g) obtained in this study are in good agreement with the recommended values.
Nakajima, Kunihisa; Omichi, Toshihiko*; Arai, Yasuo
Journal of Nuclear Materials, 304(2-3), p.176 - 181, 2002/08
Times Cited Count:3 Percentile:23.41(Materials Science, Multidisciplinary)The oxygen potentials of two type urania-yttria solid solutions, one is the solid solution expressed as nearly U Y
Y O
O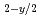 and the other is nearly stoichiometric solid solution, are investigated by use of a mass spectrometer combined with a Knudsen cell. The oxygen potentials for the nearly stoichiometric specimens are much higher than those for pure urania with similar stoichiometry. On the other hand, the oxygen potentials for fully reduced specimens almost agree with those for pure urania having the same oxygen deficiencis.
and the other is nearly stoichiometric solid solution, are investigated by use of a mass spectrometer combined with a Knudsen cell. The oxygen potentials for the nearly stoichiometric specimens are much higher than those for pure urania with similar stoichiometry. On the other hand, the oxygen potentials for fully reduced specimens almost agree with those for pure urania having the same oxygen deficiencis.